Characteristics
category
Platform
Comfort and design
Handling and systems control
Safety
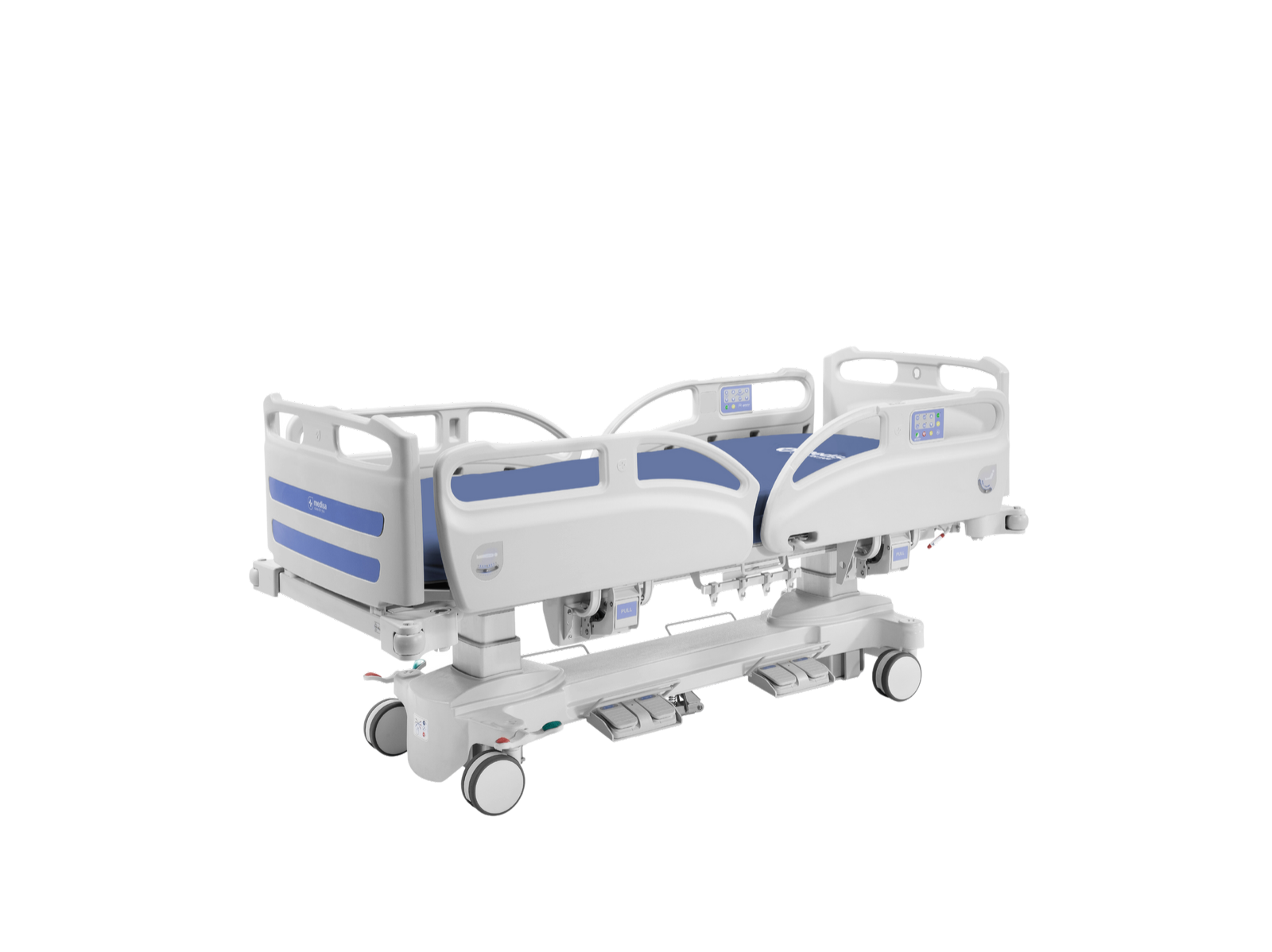
Epoxy coated steel tube frame.
New design for headboard and footboard that improves matress retention, preventing its displacement. The fixing system to the structure allows detaching them easily without tools.
Complete with supports for accessories (IV drip rod, lifting pole,etc. ) on each corner of the bed.
Raising system for greater stability in any position and working load up to 250 kg. SGS certificated.
Bed base with four independent and detachable HPL lying sections. Useful surace 100%.
Adjustable side retainers that prevent displacement of mattresses of different sizes.
Four independent HDPE Medical side rails in accordance with standard rule EN 60601-2-52 with gas spring assist and one hand operation
Side rails with an innovative mechanism that requires minimal folding, no more than 7.5 cm
The design of the side rails allows protecting the patient in the totality of the lying surface
“Help to stand up” button. It allows optimizing the sequence of thepatient’s exit from the bed
The bed has two blue LED light points integrated in the rail that are activated when it is lowered
Automatic alarm of the backrest at 30º
Double automatic anti-decubitus regression system to allow a backplane regression (11 cm) and leg regression (7 cm)
Radiolucent backrest section with RX cassette holder (optional Ref.CHV26) that allows the extraction of the tray without articulating the backrest
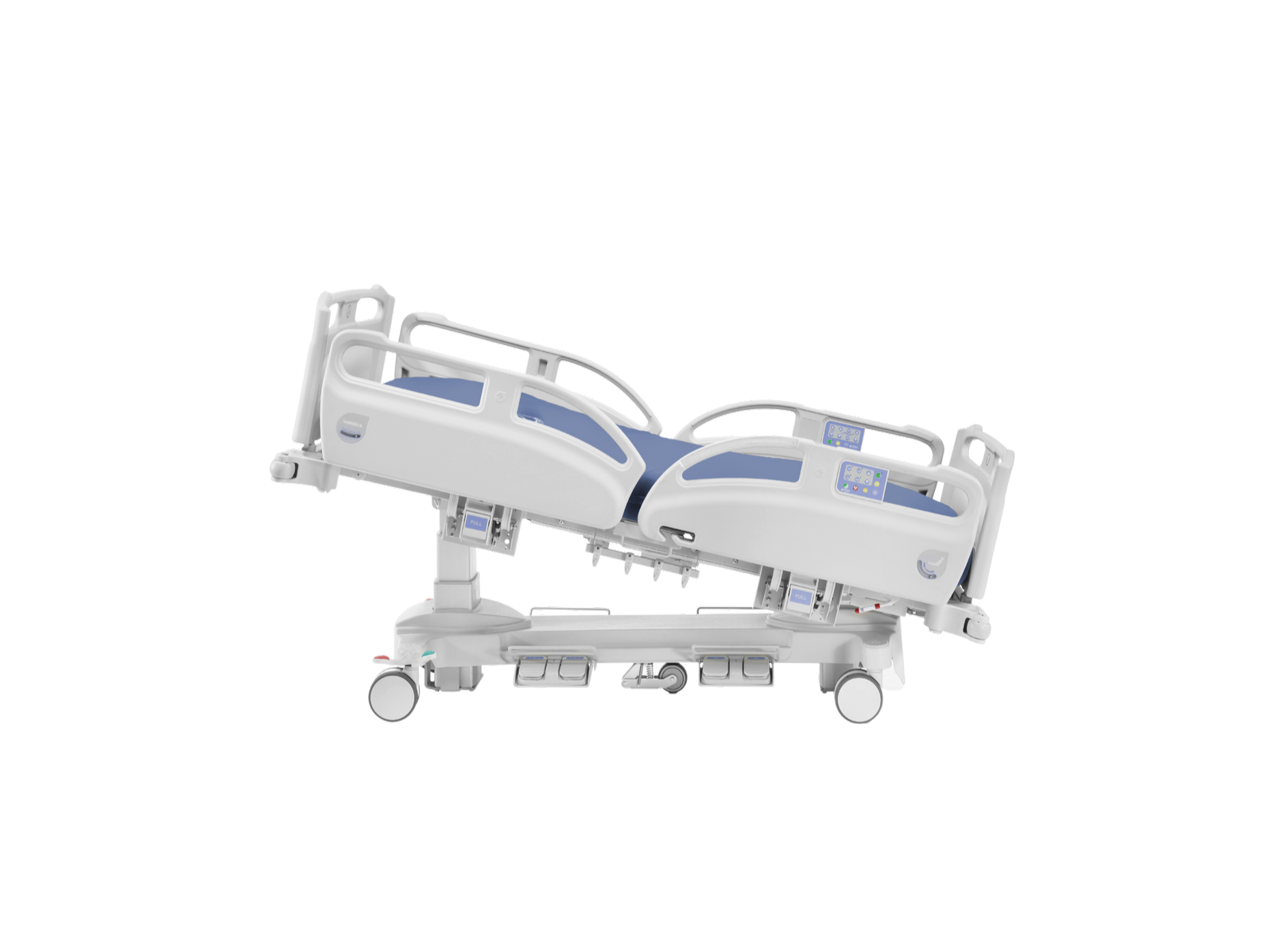
Sections, height and trendelenburg / reverse trendelenburg adjustment accomplished by low tension motors electric motors
Independent foot angle from knee angle by mechanical activation of 5 positions providing a greater ergonomics
Cardiac chair position to provide a more comfortable patient stay
Exit position button, that easies the patient to leave the bed
Functions can be locked individually or in a simultaneous way through “STOP” button
Centralized braking system and steering facility
5th wheel (optional Ref.CHV24) central and directional Ø125 mm. 360º rotating with two gases that adapt to uneven ground
Panel for medical staff, it allows to activate and cancel the different functions of the bed
Patient handset (inside rails): Allows patients to control and regulate the sections of the backrest, back/foot and height, activation of the “GO” button and the night light under the bed
Emergency release system in the event of electrical or battery back-up failure (CPR position)
Bilateral pedal for height adjustment. Allows adjust height to reduce harm risks
Double led safety positioning light (green color to indicate minimum height, red when it exceeds minimum safety height)
Braking pedal alarm: When the bed is connected this alarm warns if the braking system is deactivated
Low battery alarm
Safe fixation of the footboard and headboard that can be locked by a manual fixation system, without tools, which allows the bed transport without gaps and risks
Anti-trapping sensor with automatic engine stop when
detecting an obstacle
IPX6 protection against water and dust
Technical file
Technical data
- External dimensions: 2233 x 980 mm
- Patient surface: 2000 x 850 mm
- Height adjustment: 428 to 748 mm (455 mm to 775 mm with scale)
- Backrest tilting: 67º
- Legrest tilting: 28º
- Footrest tilting: 19º
- Trendelenburg/reverse-trendelenburg: ± 16º
- Maximum working load: 250 kg
- Weight without accessories : 150 kg
- Backrest autoregression: 150 mm
- Footrest autoregression: 70 mm
Electrical features
- Power supply: 240-100V 50-60Hz
- Maximum current intake: 5A, 100-240V
- Protection indicator: IP66
- Class protection: Clase II
- Electric shock protection: TYPE B
Accessories
Optional accessories
- Linfting pole (REF. CHA17)
- IV Pole (REF. CHA15)
- Mattress
Optional equipment
- Headboard fixed to the base (REF. CHV65)
- Bilateral pedal with locking function for height adjustment or examination position (REF. CHV83)
- Fifth wheel (REF. CHV 24)
- Brake alarm (REF. CHA30)
- X-ray cassette holder guides (REF. CHV26)
Normative
All products manufactured by MEDISA comply with Directive 2007/47/EEC on medical devices (Annex I and Annex VII).
In the same way, all its processes have been evaluated and certified according to the requirements of the UNE-EN ISO 9001: 2015 standard for Quality Management, the UNE-EN ISO 14001: 2015 standard for Environmental Management and the UNE-ENE ISO 13485: 2016 standard for Quality Management of medical devices.
MEDISA products are subject to EN-ISO 14971, EN-ISO 15223-1, EN-ISO 13485, EN 60601-1, EN 60601-1-2, EN 60601-2-52, EN 60601-1-6, EN 60601-1-8, EN 62366.
Certified by CSQ: CERTIFICATES Nº 9120.MDIB, Nº 9191.MDI2, Nº 9124.MIBE.
Scope: design, manufacture, marketing and technical assistance of hospital beds and armchairs. Marketing of hospital furniture.