Medisa, within the framework of the ICEX Next Program, has received support from ICEX and co-financing from the European FEDER fund. The purpose of this support is to contribute to the international development of the company and its environment.
Characterestics
It does not present medical contraindications, nor elements that are harmful, harmful or toxic to health.
Maintains memory moments after removing pressure
Oko-tex Standard 100 certified
Latex Free
Can be manufactured for morbid obesity
It adapts perfectly to articulated, hospital and geriatric beds, it does not interfere with the use of railings.
Ergonomic and with Biocell technology
Avoids pressure points and distributes support points, which helps improve blood circulation
Technical file
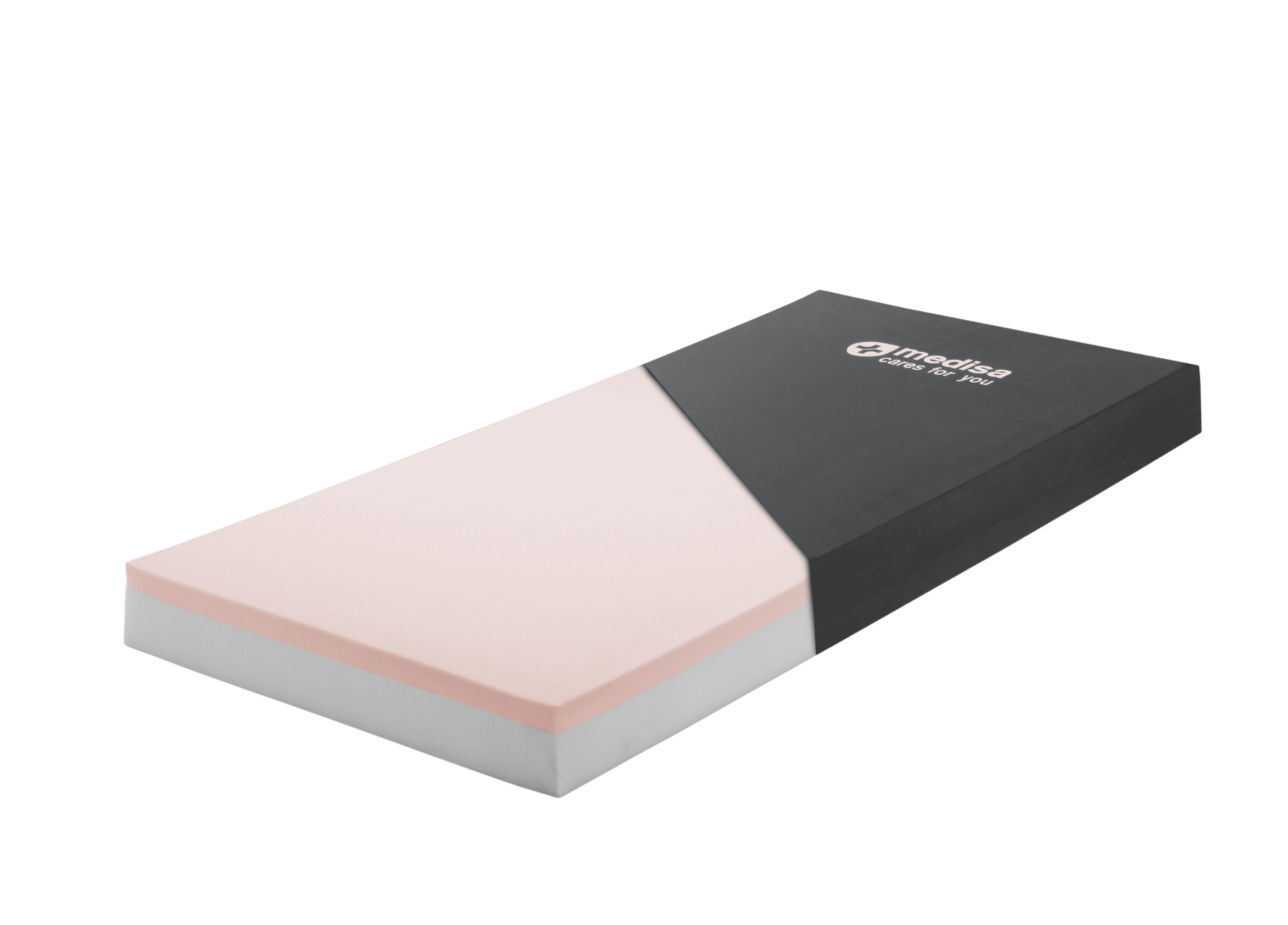
Mattress technical data
Dimensions: 190 x 85 x 13 cm. Available in other sizes and thicknesses (consult)
Upper part: 4 cm memory foam with HR 50 density
Bottom: 9 cm polyurethane foam with HR 30 density
Includes model CLO15 case
Case characteristics
Elastic in all directions
Washable at 95º without shrinkage and resistant to chemical disinfection and autoclave sterilization up to 120º.
Fireproof, waterproof and breathable fabric, avoiding excess temperature and humidity
Easy-to-clean anti-bacterial and anti-fungal finish
Non-slip bottom
Hydrostatic pressure
L-shaped zipper for easy donning and flap
Latex free and anti-allergic
Normative
category
All products manufactured by MEDISA comply with directive 2007/47/EEC on medical devices (Annex I and Annex VII)
In the same way, all its processes have been evaluated and certified according to the requirements of the UNE-EN ISO 9001:2015 Quality Management standard, the UNE-EN ISO 14001:2015 Environmental Management standard and the UNE-ENE ISO 13485:2016:2016 Quality Management of health products standard
MEDISA products are subject to regulations EN-ISO 14971, EN-ISO 15223-1, EN-ISO 13485, EN 60601-1, EN 60601-1-2, EN 60601-2-52, EN 60601-1-6 , EN 60601-1-8, EN 62366
Certified by CSQ: CERTIFICATES No. 9120.MDIB, No. 9191.MDI2, No. 9124.MIBE
Reach: design, manufacturing, marketing and technical assistance of hospital beds and chairs. Marketing of hospital furniture