Medisa, within the framework of the ICEX Next Program, has received support from ICEX and co-financing from the European FEDER fund. The purpose of this support is to contribute to the international development of the company and its environment.
Characteristics
Generals
- Made of highly resistant and durable 30 x 30 x 1.5 steel tube protected with epoxy paint with 80 micron anti-corrosion protection
- Metal arms upholstered in self-extinguishing vinyl fabric according to Standard M1, antibacterial, resistant to stain absorption
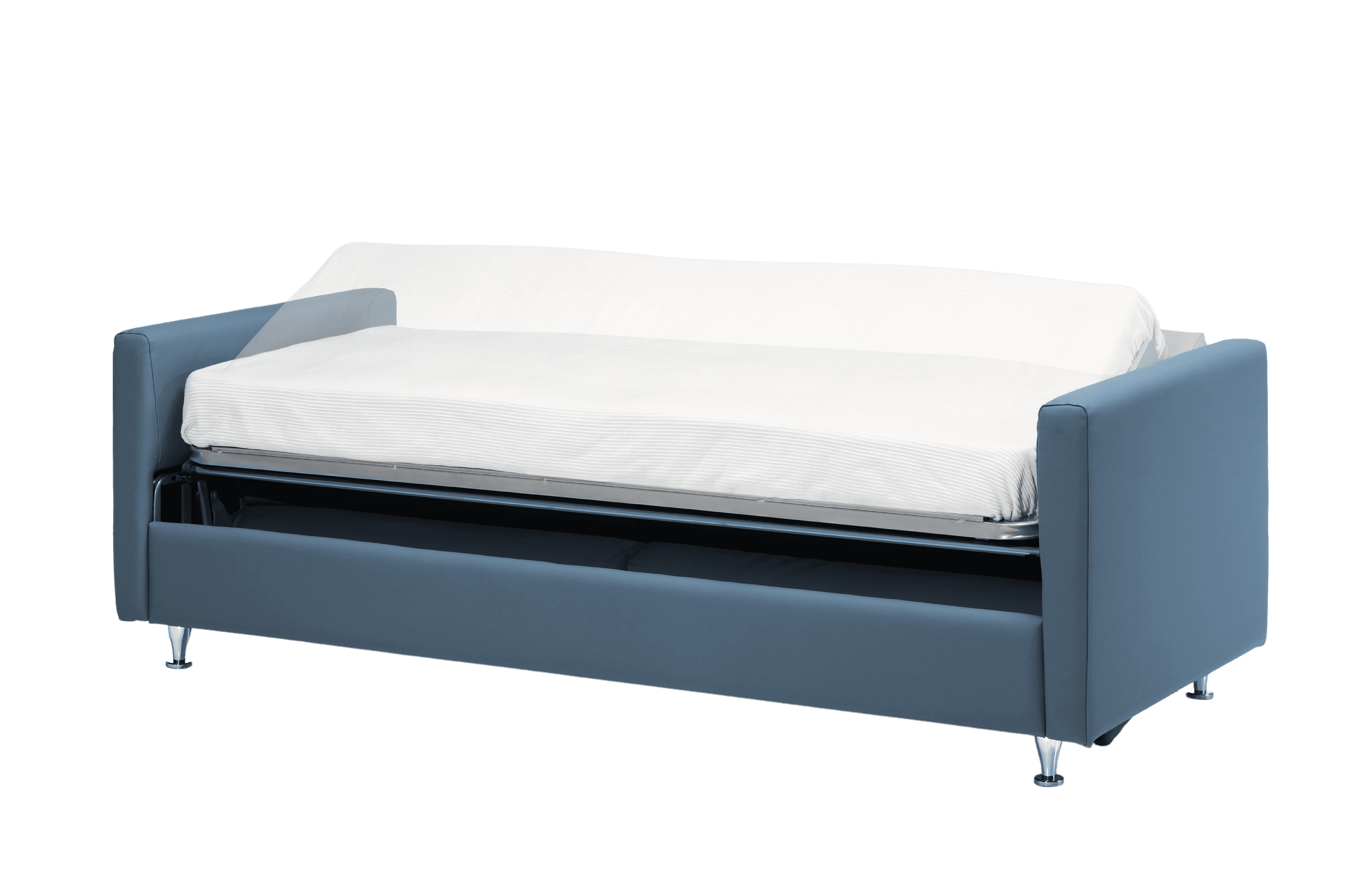
Structure on legs. Optional with wheel
- The system is designed to store two sheets, a quilt and a pillow inside.
- Minimalist design, with straight lines. With straight arms that allow for comfortable and easy cleaning and maintenance
- Seat cushions covered in high-density foam rubber 35 kg
- Robust, reliable and simple bed exit mechanism, avoiding worker fatigue
Upholstery
- Layer composition: 100% vinyl
- Support composition: 100% polyester with a weight of 650 gr/m2.
- Permablock protective layer, anti-germ protection (antimicrobial, antibacterial, antifungal)
- High level of abrasion resistance: >300,000 cycles (Martindale)
- Low temperature resistance: – 23 º C.
- Light resistance: 1000 hours, blue wool scale
- Fireproof protection
- Cleaning with a sponge and neutral soap or with a damp cloth
- Fully removable upholstery
Mattress
- It is a thermosensitive foam
- Prevention of bedsores or pressure ulcers
- Composed of a lower layer of high-resistance foam, which acts as support for the patient’s weight, and another upper layer, which is attached to the body of the patient or companion using heat and pressure
Case
- Waterproof, breathable, water-repellent, latex-free, fire-retardant, fire-resistant cover (BS 7175 Standard), anti-fungal, anti-bacterial, sliding surface for easy cleaning
- Fabric composition:
Underside: Fabric – 105 g/m2 – 100% polyester
Top side: 75 g/m2 – 100% polyurethane
Total weight: 180 g/m2 - L-shaped zipper for easy donning and flap
Technical file
Technical data
- Exterior measurements: 205 cm wide x 85 cm deep
- Open bed measurements: 205 cm wide x 85 cm deep x 60 cm high
- Visco mattress density: 50 kg
- HR foam density: 25 kg
- Mattress height: 10 cm
- Mattress measurements: 80 x 190 cm
Normative
All products manufactured by MEDISA comply with directive 2007/47/EEC on medical devices (Annex I and Annex VII).
All processes have been evaluated and certified according to the requirements of the UNE-EN ISO 9001:2008 standard for Quality Management and the UNE-EN ISO 14001:2004 standard for Environmental Management, and the UNE-EN ISO 13485 standard for medical devices. The quality management system.
The product complies with the new European Regulation 745/2017 on medical devices and controlled under the EN ISO 9001:2015 quality management system