Medisa, within the framework of the ICEX Next Program, has received support from ICEX and co-financing from the European FEDER fund. The purpose of this support is to contribute to the international development of the company and its environment.
Characteristics
category
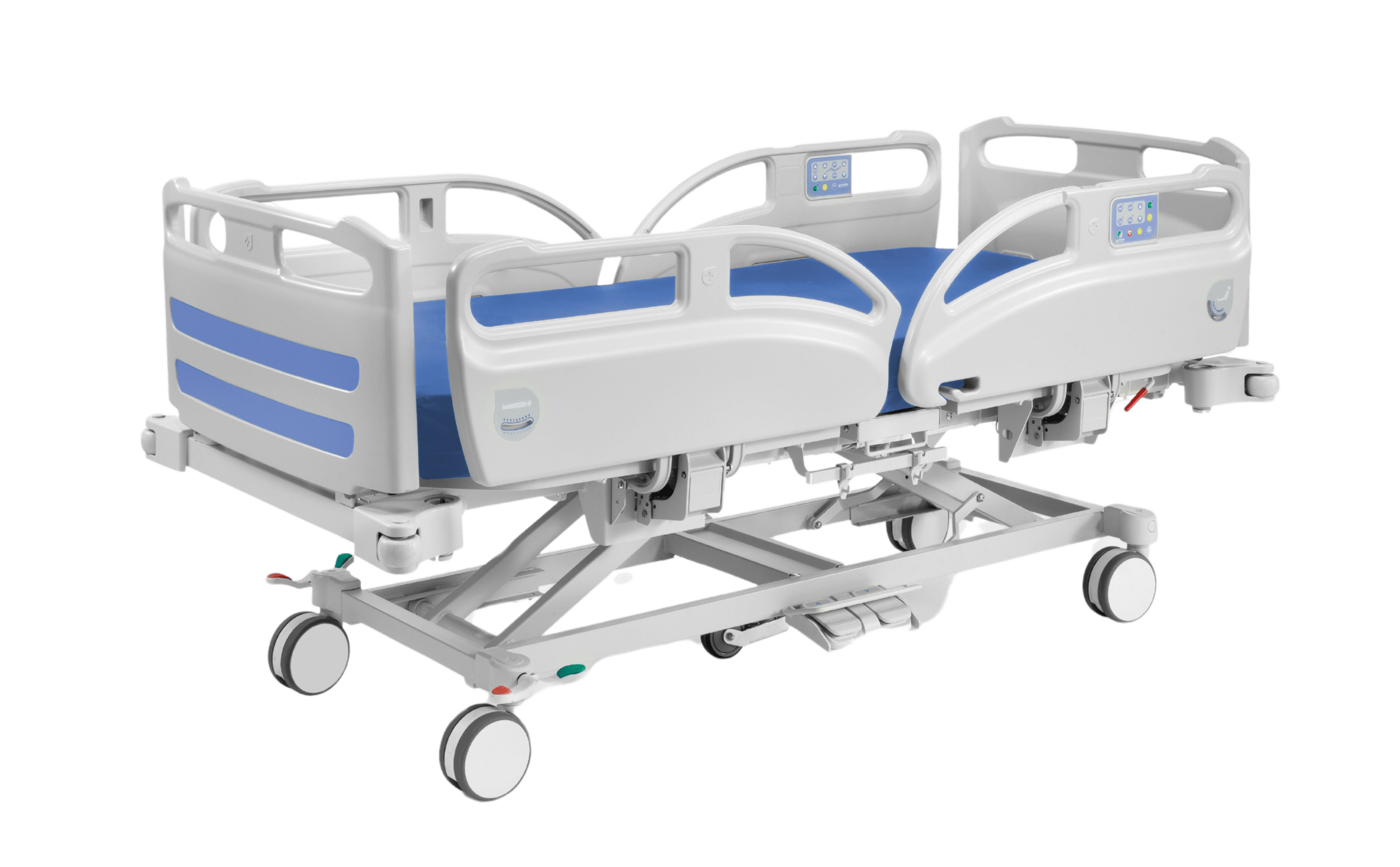
Mattress base
Design and comfort
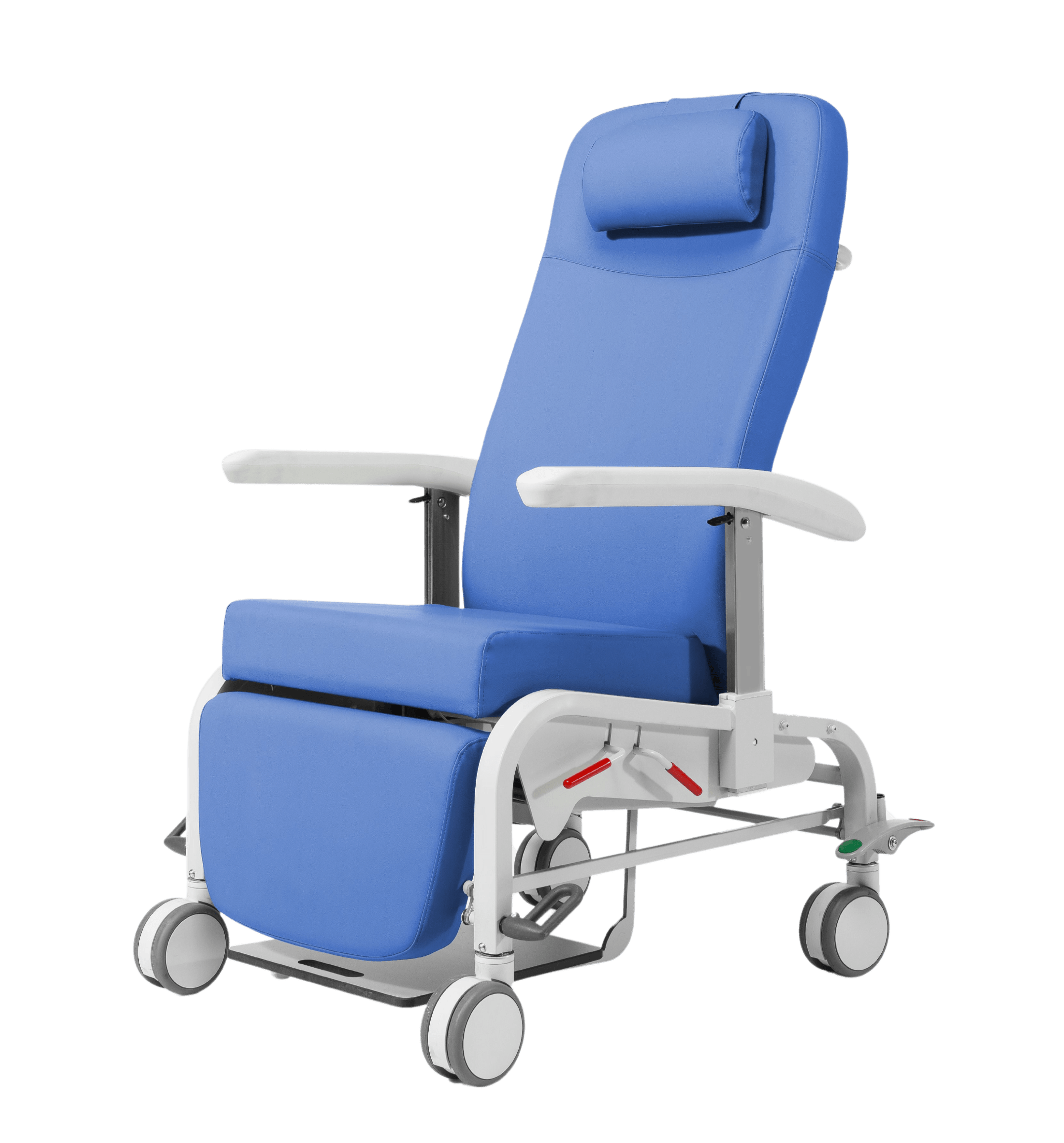
Handling
Safety
Structure made of steel tube with epoxy coating
Headboard and footboard fixing system, without the need for tools
Lateral arches, which prevent displacement of the mattress
Lifting systems supported by compasses that provide great stability in any position and allow a load of up to 250 kg. SGS certified
Four removable modules made of ABS. 100% useful area
Optional: Integrated bed extension with bedding support (±20cm.)
Optional: Radiolucent bed for the plane of the trunk with optional chassis holder (Ref.CHV26)
Anti-decubitus system, in the trunk plane, offering protection against pressure ulcers
Articulation of the bed base planes using low voltage electric motors, complying with EN 60601-1 regulations.
Foot plane independent of the knee plane with mechanical articulation, providing greater ergonomics
Height elevation from 41.4 cm to 80.5 cm by electric drive
Centralized brake system and servo steering. Ø150mm double band wheels
Manual keypad with pictograms for easy reading and handling
Bed Function Cancellation Device
Thermoplastic lower fairing that protects the bed mechanisms and facilitates cleaning
Split handrails in ABS (4) complying with regulations 60601-2-52
Optional: Set of metal railings
Emergency release system of the trunk plane that allows a rapid descent of the plane in case of emergency, battery failure or power failure
Technical file
Technical data
- External dimension: 2130 x 960 mm
- Patient surface dimension: 2000 x 870 mm
- Height adjustment: 415- 805 mm
- Backrest tilt: 65º
- Legrest tilt: 33º
- Maximum weight load: 250 kg
- Weight of the bed without accessories: 150 kg
- Backrest section auto-regression: 110 mm
- Legrest section auto-regression: 40 mm
Electrical characteristics
- Power supply: 110-230V 50-60Hz
- Maximum Consumption: 3.15A, 230 Volt
- Protection indicator: IP X4
- Protection class: Class II
- Degree of protection against discharge: TYPE B
Accessories
Optional accessories
- Set of metal or ABS railings
- Integrated bed extension with bedding holder
- Radiolucent bed for the backrest section with optional chassis holder
Normative
All products manufactured by MEDISA comply with directive 2007/47/EEC on medical devices (Annex I and Annex VII)
In the same way, all its processes have been evaluated and certified according to the requirements of the UNE-EN ISO 9001:2015 Quality Management standard, the UNE-EN ISO 14001:2015 Environmental Management standard and the UNE-ENE ISO 13485 standard. :2016 Quality Management of health products
MEDISA products are subject to the standards EN-ISO 14971, EN-ISO 15223-1, EN-ISO 13485, EN 60601-1, EN 60601-1-2, EN 60601-2-52, EN 60601-1-6 , EN 60601-1-8, EN 62366
Certified by CSQ: CERTIFICATES Nº 9120.MDIB, Nº 9191.MDI2, Nº 9124.MIBE
Scope: design, manufacturing, marketing and technical assistance of hospital beds and chairs. Marketing of hospital furniture