Medisa, within the framework of the ICEX Next Program, has received support from ICEX and co-financing from the European FEDER fund. The purpose of this support is to contribute to the international development of the company and its environment.
Characteristics
category
Structure
Mobility and comfort
Design and ergonomics
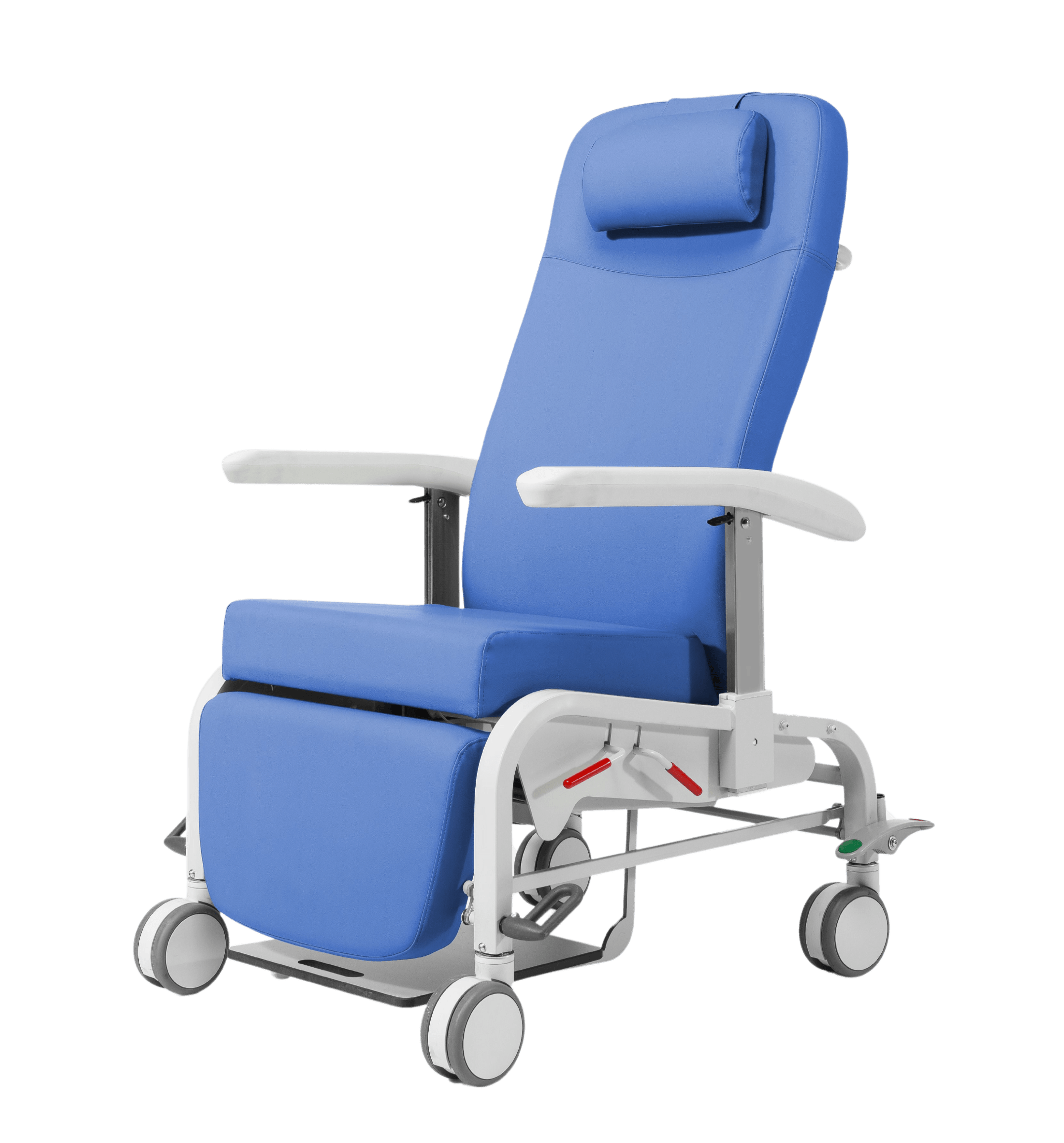
Steel frame with a high-quality epoxy finish.
Anticorrosive and antimicrobial treatment (optional)
Ensures high resistance to impacts and friction
Anatomical backrest and lumbar support, thanks to the ergonomic backrest system
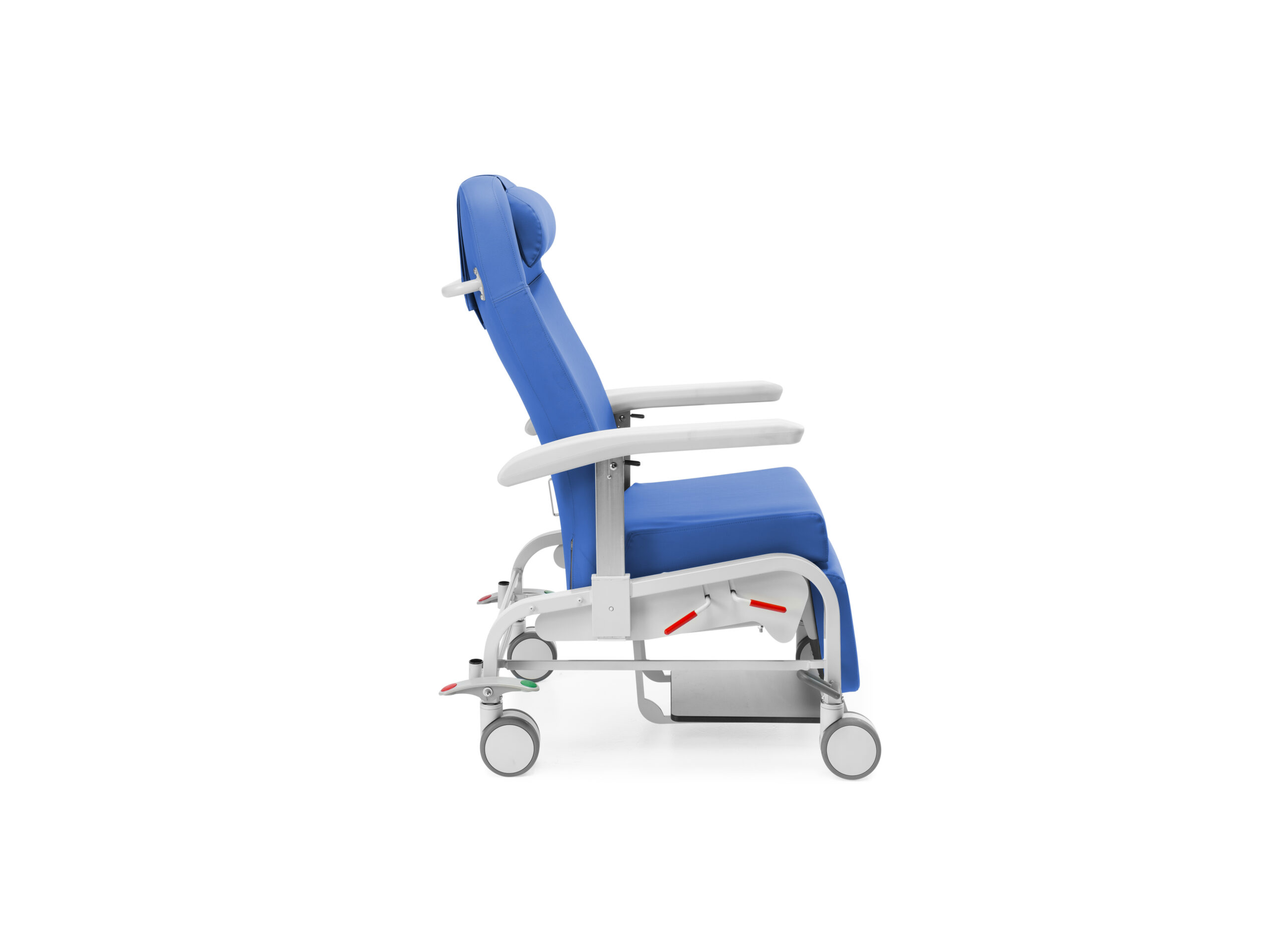
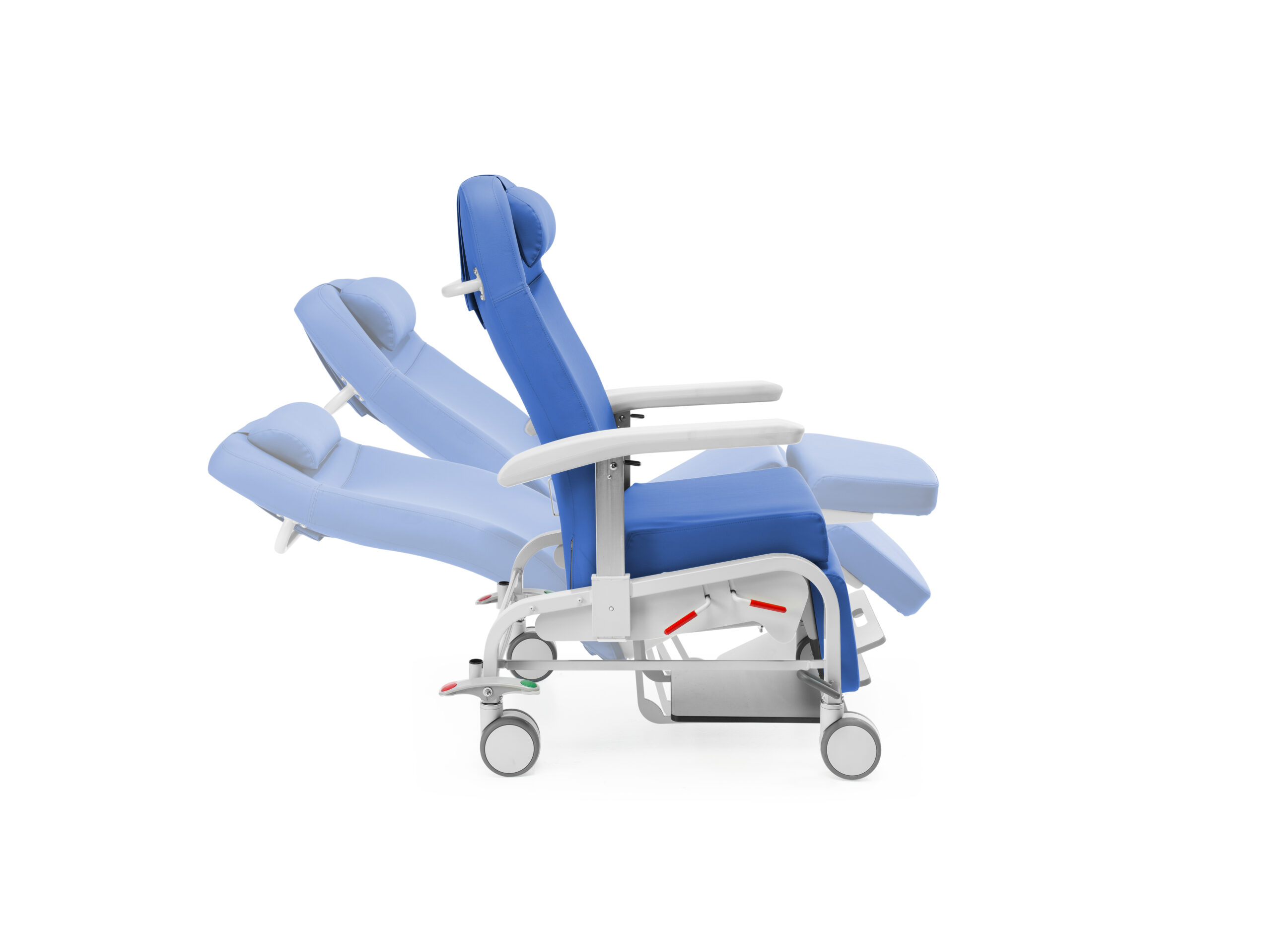
Movement with an independent mechanism for articulating the backrest and legrest, pneumatically actuated on both sides by a gas spring, minimizing the activation force required for the mechanism. Automatic return to the chair position
Easy-access levers for the patient seated on both sides of the chair and locking system
Backrest with Bloc-o-Lift system, which allows it to be operated in multiple positions and nailed in any intermediate position
“Zero gravity” tilt system, avoiding pressure in the lumbar area
The legrest is foldable and retractable under the seat
With four Ø 125 mm wheels and centralized braking system activated by external bar and bilateral pedals
Integrated footrest, extendable and retractable, with anti-tilt system, which is stored under the patient’s seat
Adjustable armrest in 5 different heights, made of high-quality PUR, resistant and durable, with an ergonomic, curved design and soft touch to ensure greater comfort.
All edges are rounded without sharp edges and without cutting surfaces
High-size anatomical backrest, 84 cm and with lumbar reinforcement
Carrying handle on the backrest for easy transportation
Smooth surfaces and compact material allow for quick and easy cleaning and decontamination.
High-density foam padding.
Smooth, non-porous upholstery made of self-extinguishing vinyl fabric with a waterproof, antimicrobial, antibacterial, and antifungal protective layer that is highly abrasion-resistant.
Technical file
Dimensions
- Dimensions: 1225 x 690 x 860 mm
- Backrest dimensions: 840 x 520 mm
- Seat dimensions: 510 x 500 x 530 mm (it depends of the wheel and thickness of the upholstery)
- Legrest dimensionss: 510 x 320 mm
- Extendable footrest: 300-355 x 390 mm
- Pur armrest dimensions: 700 x 670 mm.
- Armrest height: 530-735 mm
Technical data
- Backrest tilt: 80º – 180º ( (Lying position horizontal up to 180º)
- Legrest tilt: 0º – 90º
- Maximum weight load: 180 kg
- Weight without accessories: 45 kg
*Factory tolerance +/- 1cm. (Measurements may vary depending on the thickness of the upholstery and the diameter of the wheels)
NOTE: The measurements and characteristics of the products presented may be modified without prior notice.
Accessories
Included
Bilateral supports for diuresis bags, drains… integrated into the structure of the chair
Bilateral supports for IV supports
Optional
Roll holder support
Auxiliary tray
IV pole support
Normative
All products manufactured by MEDISA comply with directive 2007/47/EEC on medical devices
All processes have been evaluated and certified according to the requirements of the UNE-EN ISO 9001:2008 standard for Quality Management and the UNE-EN ISO 14001:2004 standard for Environmental Management, and the UNE-EN ISO 13485 standard for medical devices