Medisa, within the framework of the ICEX Next Program, has received support from ICEX and co-financing from the European FEDER fund. The purpose of this support is to contribute to the international development of the company and its environment.
Characteristics
category
Mattress Base
Comfort
Handling
Safety
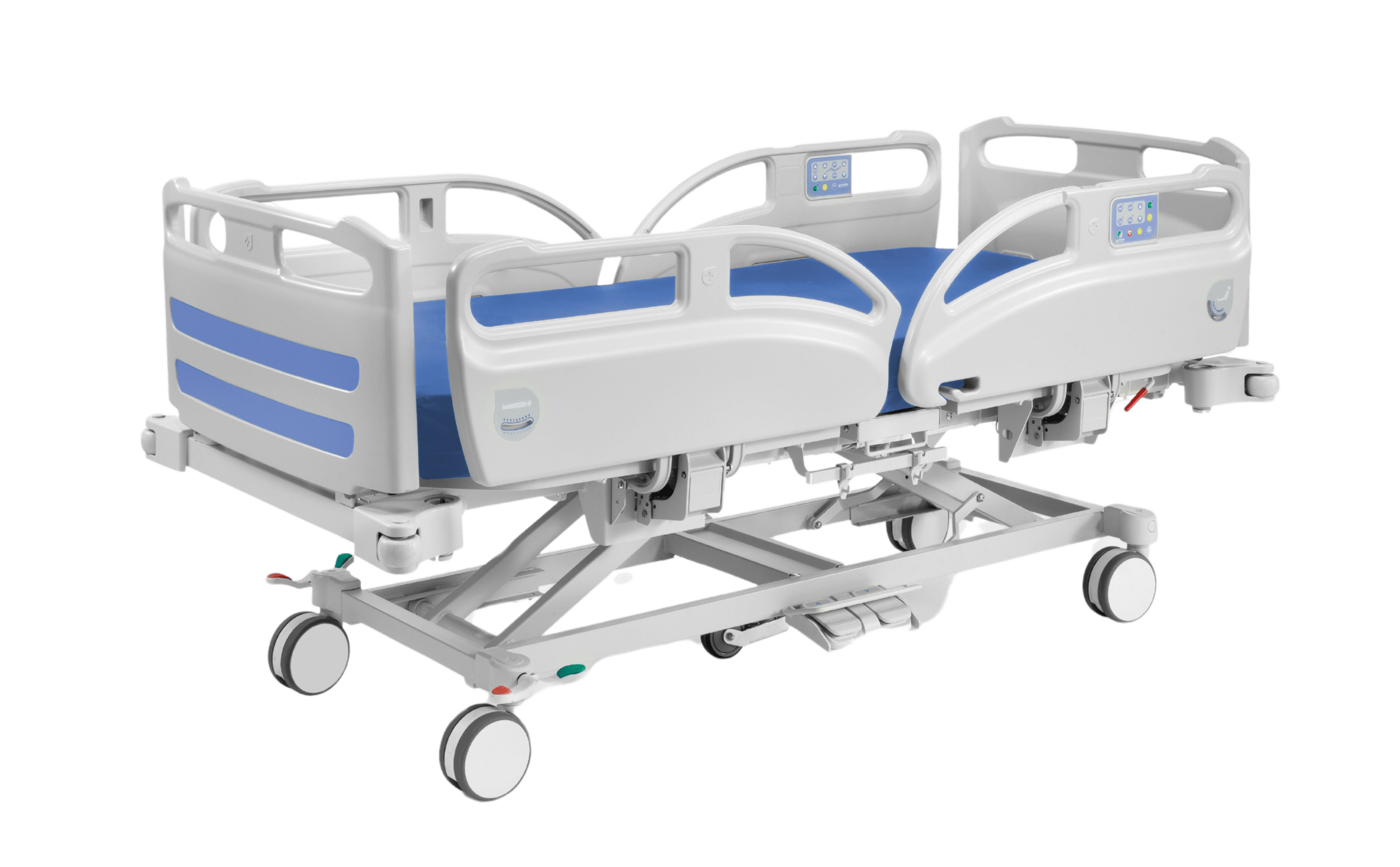
Structure made of steel tube with epoxy coating
Bed composed of 4 sections (3 articulated and 1 fixed) made of compact HPL material
Resistant to moisture and impact, allowing for simple cleaning
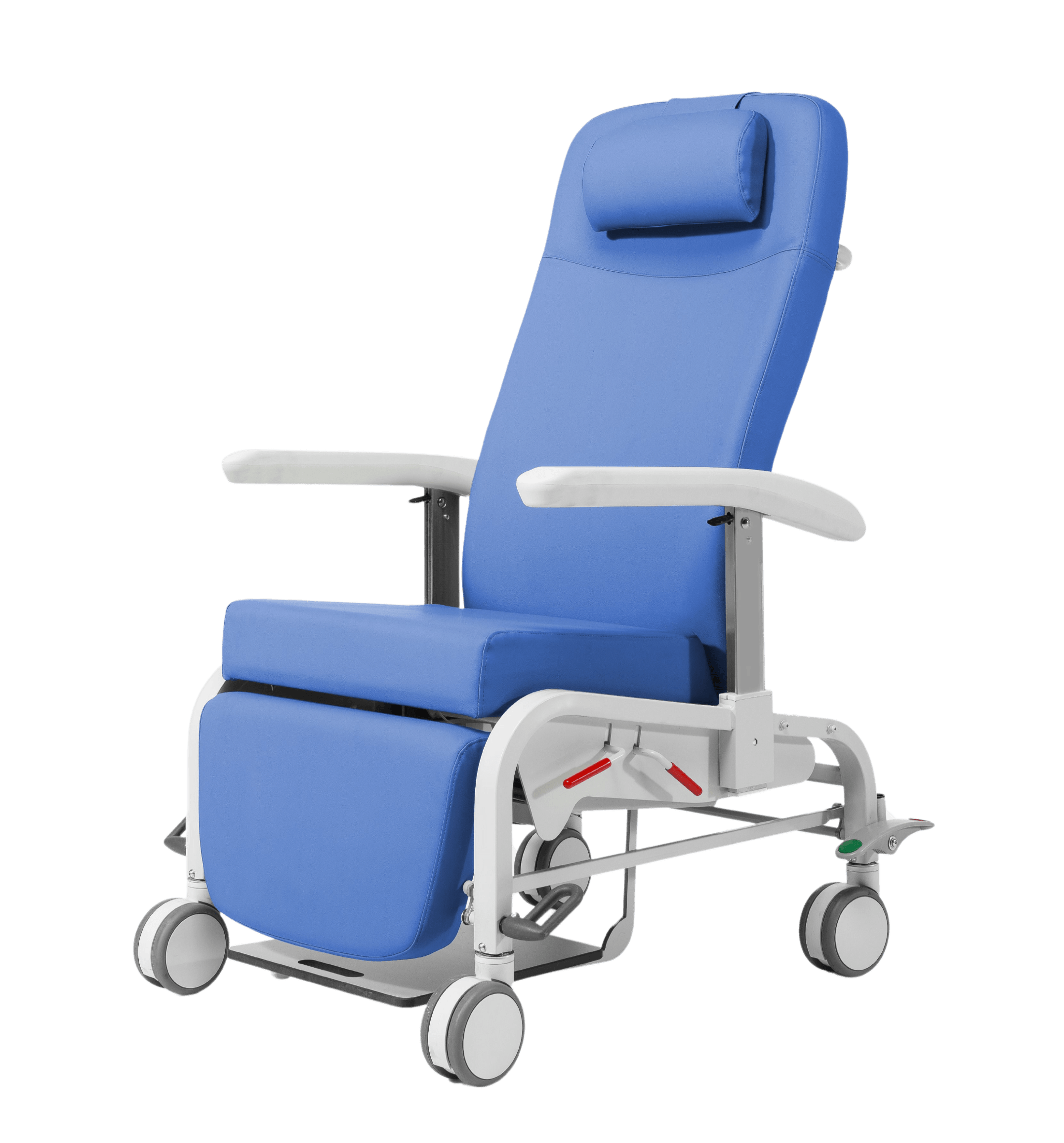
Cardiac chair position
Fully transparent radio bed base
Useful area 100%
Support for additional IV poles (depending on size) in the corners
Urine bag hooks on each side of the stretcher
X-ray permeable mat, 10 cm thick
Padding made of high-density polyurethane foam, designed for optimal comfort and ergonomic suitability
Upholstered in self-extinguishing vinyl fabric
With four 200mm diameter, antistatic wheels and a wheel with servo steering
Centralized brake and directional lock via pedal located on each wheel
5th wheel diameter 125 mm servo assisted with gas spring
Folding handles to facilitate handling of the stretcher during transfer
Articulation of the trunk plane by pneumatic operation up to an angle of 90º
Height elevation from 515 mm to 820 mm using hydraulic columns
Hip and leg articulation by means of gas springs by bilateral levers, which provides greater ergonomics
Trendelemburg / Reverse trendelemburg ±16º by hydraulic drive
Bilateral pedal for hydraulic height adjustment and for Trendelenburg and Reverse trendelenburg control
Protective bumpers located in the corners
ABS lower fairing for easy cleaning, with built-in oxygen bottle holder
Folding side rails 150 long x 34 cm high; 29 cm distance between vertical bars
Easy and safe handling, without risk of bruising or entrapment
Its lowest position does not exceed the height of the mat
Technical file
Dimensions
- External dimension: 204 x 89 cm
- Patient surface dimensión: 189 x 68 cm
- Backrest dimensions: 620 x 710 mm
- Legrest dimensions: 660 x 1160 mm
Technical data
- Height adjustment: 515 – 820 mm
- Height adjustment with chassis support: 570 mm – 880 mm
- Backrest tilt: 90º
- Trendelenburg/Reverse trendelenburg: ± 16º
- Maximum weight load: 317 kg
- Weight without accessories: 100 kg
Accessories
Included
- Mattress
- Foldable and height adjustable IV pole
Optional
- Monitor support
- Paper roll holder
- Transfer mat
Normative
All products manufactured by MEDISA comply with directive 2007/47/EEC on medical devices (Annex I and Annex VII).
All processes have been evaluated and certified according to the requirements of the UNE-EN ISO 9001:2008 standard for Quality Management and the UNE-EN ISO 14001:2004 standard for Environmental Management, and the UNE-EN ISO 13485 standard for medical devices.
The product complies with the new European Regulation 745/2017 on medical devices and controlled under the EN ISO 9001:2015 quality management system.