Medisa, within the framework of the ICEX Next Program, has received support from ICEX and co-financing from the European FEDER fund. The purpose of this support is to contribute to the international development of the company and its environment.
Characteristics
category
Mattress Base
Comfort
Handling
Safety
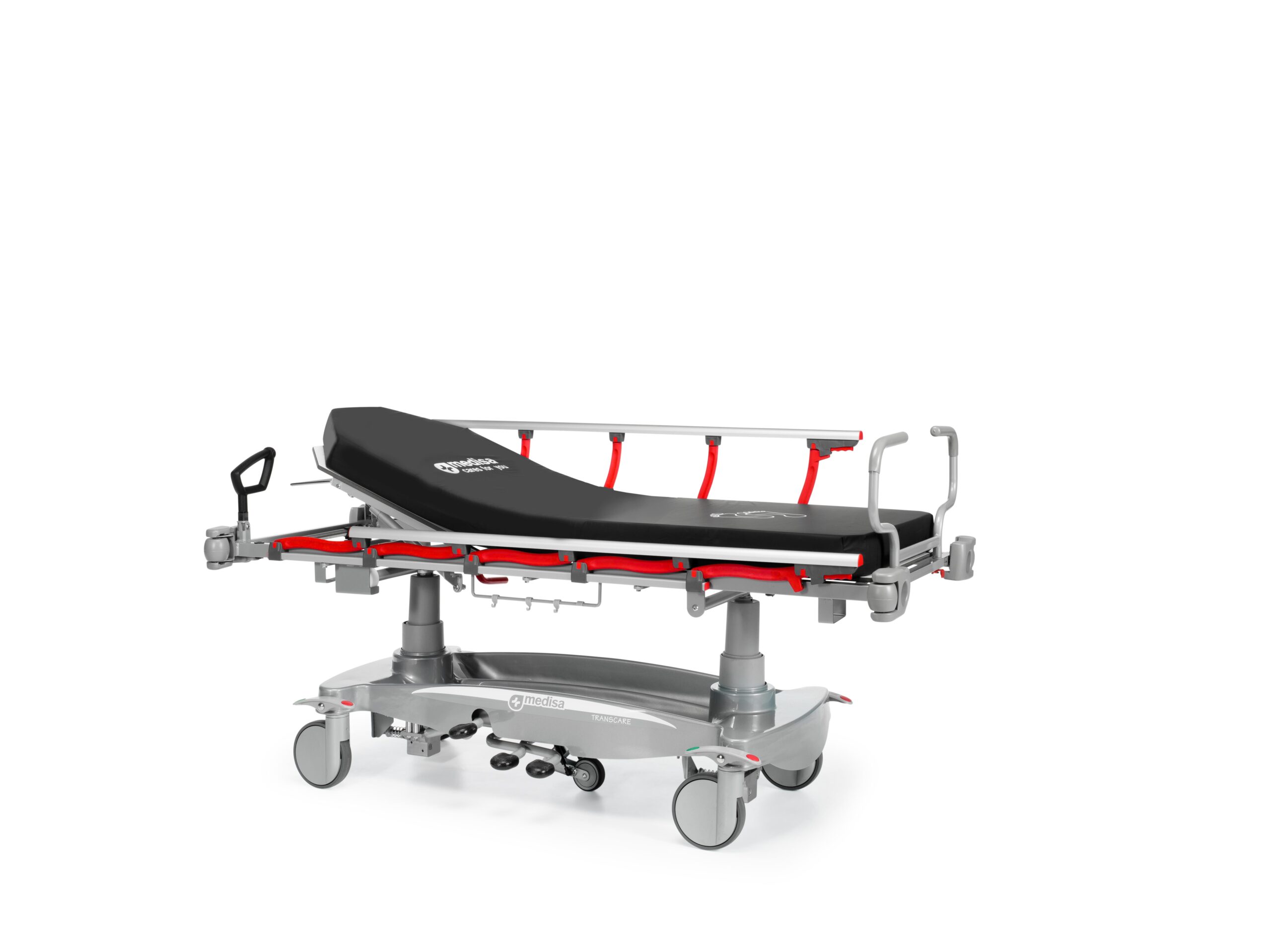
Structure made of steel tube with epoxy coating
Bed made up of two sections with articulated backrest, made of high-qualityand durable HPL compact material. This material offers excellent resistanceto deformation, impact and wear, as well as being completely permeable toX-rays
Base with ABS cover protecting the structure and cylindrical trims coatingpumps, for easier cleaning
Support for additional IV poles (depending on size) in the corners
Urine bag hooks on each side of the stretcher
Longitudinal sliding tray that allows inserting of a chassis-holder plate forcarrying out radiographic tests (optional accessory Ref. MBTR051) Forcassettes up to approximately 60 x 45 cm.
Manual backrest adjustment through two gas spring actuators
of 200 NW each
Height adjustment system: using two hydraulic column pumps,which allow operation from both sides of the stretcher with a bilateral pedal
Comfortable access for patients: The low height of the stretcherprovides greater comfort and makes the stretcher easier to use forboth the patient and medical staff.
Adjustable positions:
Trendelenburg/Antitrendelenburg ±16º hydraulic drive via bilateralpedal, allowing quick and effective adaptation to the patient’s needs
Shock position (CPR) allows the backrest to be quickly lowered inemergency situations, activated by the backrest lever.
Easy to steer and stop stretcher with centralized brake system
Four Ø200mm diameter wheels allow the stretcher to be movedcomfortably and safely
5th wheel with 100 mm diameter, directional with spring system
Facilitates turning and lateral movement, improves steering oncurves and control during transport
Brake pedals on each wheel with three positions: full brake on allfour wheels, neutral position and steer
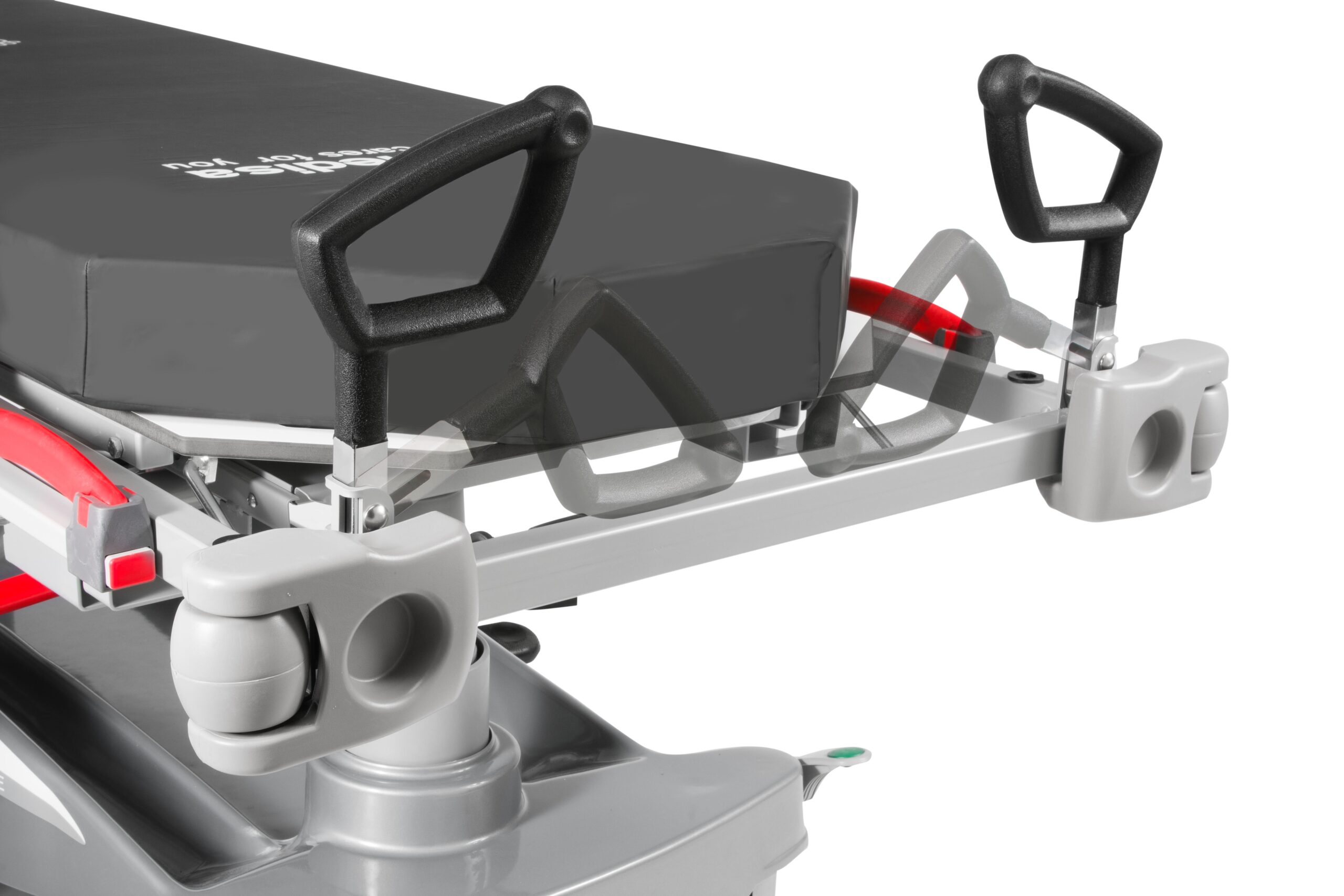
Push handles : foldable at the headboard and a detachable at thefootboard, designed to facilitate the handling of the stretchersduring their transfer. The carrying handle of the footboard isremovable and has a locking/unlocking system that prevents orallows its removal as needed
Folding and retractable side rais with safety lock and anti-entrapment system
They fold down using a push button with a double safety system inthe raised position
Its lowest position does not exceed the height of the mattress, so thetransfer is easier, more comfortable and safer
Protective bumpers located in the corners
ABS lower fairing for easy cleaning, with built-in oxygen bottle holder
Technical file
Dimensions
- External dimension: 2106x 891 mm
- Patient surface dimension: 1900 x 680 mm
- Backrest dimensions: 625×725 mm
- Legrest dimensions : 660 x 1141 mm
Technical data
- Height adjustment: 563 – 888 mm
- Backrest tilt: 90º
- Trendelenburg/Reverse Trendelenburg: ± 16º
- Maximum weight load: 310 kg
- Weight without accessories: ±100 kg
Factory tolerance +/- 1cm
NOTE: The measurements and characteristics of the products presented may be modified without prior notice.
Accessories
Included
Mattress of high-density polyurethane foam, designed for optimal comfort and ergonomic adaptation. Thickness 10 cm (optional memoryfoam mattress). Upholstered with a fireproof,antibacterial, breathable, fluid-impermeable,highly resistant and washable sanitary cover.
Telescopic IV pole
Base for oxygen pump and space for objects andbelongings
Supports for drainage and diuresis bags on both sides
Optional
Monitor holder support
Paper roll holder
Chassis holder and cassette holder support
Padding for railings
Transfer mat
Medical records support
Patient straps
Normative
The products manufactured by MEDISA comply with directive 2007/47/EEC on medical devices (Annex I and Annex VII).
All processes have been evaluated and certified according to the requirements of the UNE-EN ISO 9001:2008 standard for Quality Management and the UNE-EN ISO 14001:2004 standard for Environmental Management, and the UNE-EN ISO 13485 standard for medical devices.